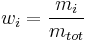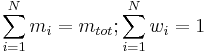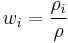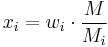Mass fraction (chemistry)
In chemistry, the mass fraction 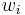 is the fraction of one substance with mass
is the fraction of one substance with mass 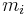 to the mass of the total mixture
to the mass of the total mixture 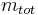 , defined as [1]:
, defined as [1]:
The sum of all the mass fractions is equal to 1:
It is one way of expressing the composition of a mixture in a dimensionless size (mole fraction is another).
For elemental analysis, mass fraction (or "mass percent composition") can also refer to the fraction of the mass of one element to the total mass of a compound. It can be calculated for any compound using its empirical formula[2] or its chemical formula[3].
Contents |
Related quantities
Mass concentration
The mass fraction of a component in a solution is the ratio of the mass concentration of that component 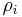 (partial density of that component) to the density of solution
(partial density of that component) to the density of solution 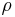 :
:
Molar concentration
The relation to molar concentration is like that from above substituting the relation between mass and molar concentration.
Mass percentage
Multiplying mass fraction by 100 gives the mass percentage, also referred to by the obsolete terms weight percent (wt%) or weight-weight percentage.
Mole fraction
The mole fraction 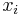 can be calculated using the formula
can be calculated using the formula
where 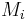 is the molar mass of the component
is the molar mass of the component 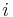 and
and 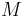 is the average molar mass of the mixture.
is the average molar mass of the mixture.
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "mass fraction".
- ^ Formula from Mass Composition
- ^ http://chemistry.about.com/od/workedchemistryproblems/a/mass-percent-worked-problem.htm
|
||||||||||||||